
CrystalBreeder
Do more with less
Leverage the capabilities of the Crystal16 and Crystalline instruments to efficiently measure primary and secondary nucleation in your research
Crystal nucleation is one of the key phenomena in crystallization. It initiates crystal formation in a running crystallization process and controls particle properties such as crystal size, crystal shape and polymorphism. The rate of nucleation dictates the evolution of subsequent crystallization phenomena like crystal growth and agglomeration. Nucleation is classified based on how it is induced. If crystalline material is already present, then it is classified as secondary nucleation. To be classified as primary nucleation, there must be an absence of crystalline material. Furthermore, primary nucleation can be classified further by whether it occurs spontaneously (homogenously) or on the surface of impurities or non-crystalline particulates (heterogeneously).
Despite its importance, nucleation still remains an inadequately understood phenomenon especially in fundamental concepts. A proper understanding of nucleation has traditionally been a difficult and time-consuming process.

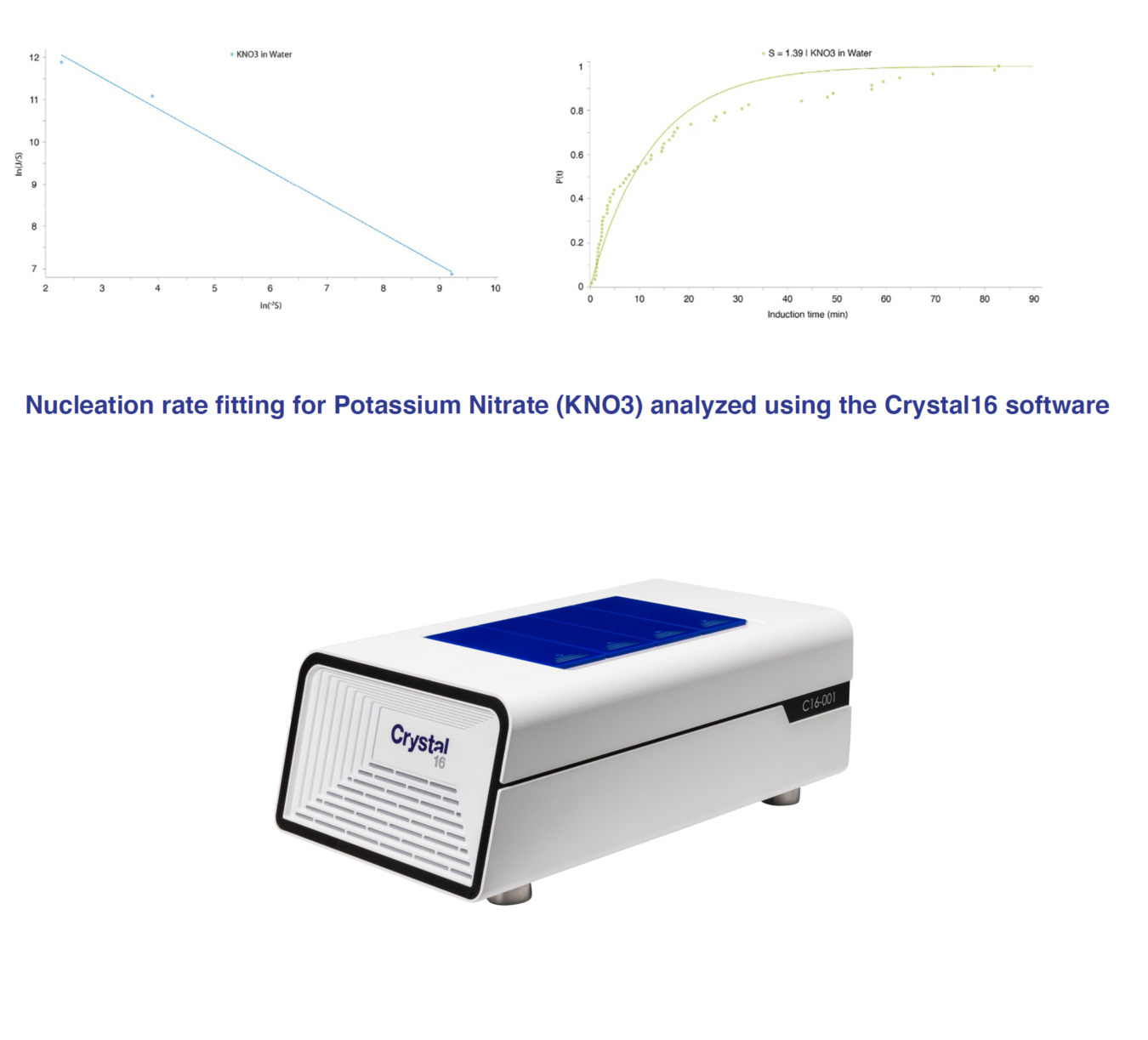
The Crystal16 offers the first commercially available software for calculating nucleation rate from the measured nucleation induction time. Coupled with the feedback control software, measuring nucleation rates has never been easier and faster.
Primary nucleation can be homogeneous (which occurs in a clear solution in the absence of crystalline material of its own kind) or heterogeneous (which occurs in the presence of impurities or foreign entities). Measurement of primary homogenous nucleation is a difficult and time-consuming process made more complex by the stochastic (random) nature of nucleation; therefore, it is imperative that temperature and thus supersaturation are tightly controlled. An additional hurdle to accurately measuring nucleation rates is that crystal nuclei are generally 1-1000 molecules in size and cannot currently be observed using available analytics. Further challenges arise from the additional processes happening alongside nucleation such as growth, agglomeration, and secondary nucleation, which can distort the measured nucleation rate.
To address the challenges in determining nucleation rate, a new method was developed using Crystal16 based on measuring the variation in induction times in stirred solution at small scale. With this method, nucleation rates have been successfully demonstrated in multiple systems in a short period of time, which can be further reduced by using Crystal16’s feedback control software feature.
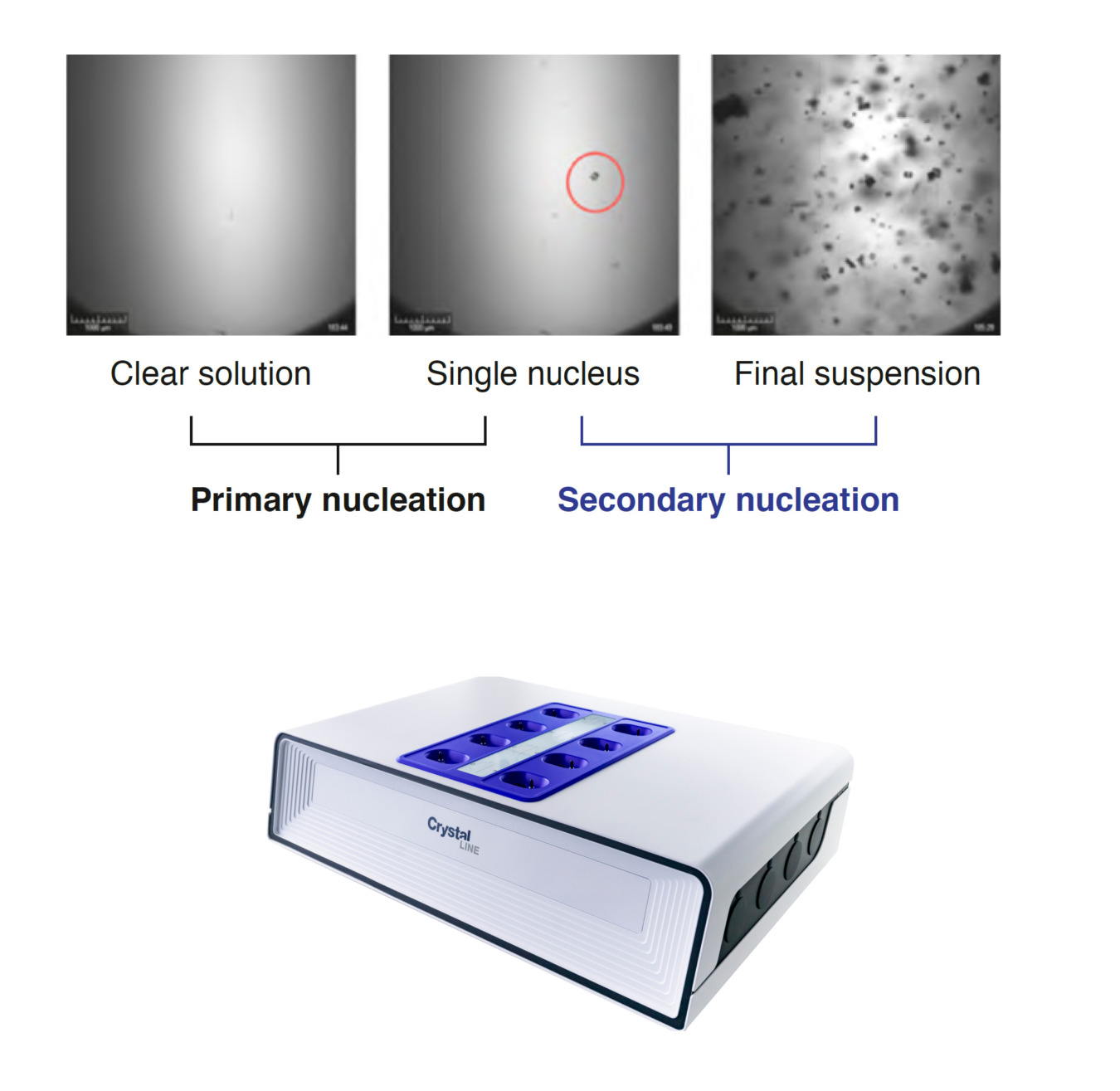
Secondary nucleation occurs as a result of the presence of crystals of the same compound in a supersaturated suspension and is typically seen after seeds are added. Secondary nucleation influences the particle size distribution of the final product and therefore affects downstream processing and particulate product quality.
The Crystalline offers the ability to quantify secondary nucleation utilizing the in situ visual monitoring, particle counter and the transmissivity measurements to identify the secondary nucleation threshold within the metastable zone width (MSZW). This approach follows the single nucleus mechanism and uses a systematic single crystal seeding method in a small batch-wise crystallization process to follow the number density of crystals during the experiment, which determines the rate of secondary nucleation rate and provides the identification of supersaturation limits at which secondary nuclei can be formed and detected.

Looking to stay ahead of the game? Design your experiments with automated decision making. Less time to optimise your process; simplify induction time and nucleation rate.