
Crystal16
The new industry standard
Efficiently track particle size, shape, morphology and polymorphic form in your drug development process, by using the unique visualization and analytical capabilities of the Crystalline device

In the manufacturing of solid dose formulations, crystallization is a commonly used process. A well-designed and controlled crystallization process can be used to substantially improve the economical and biological performance of a drug molecule through exerting control over particle size distribution and morphology, polymorphic form, and purity. Therefore, in order to maximize the benefits of a crystallization, a thorough understanding is required which can be done though modeling, design of experiment stress testing and the use of process analytical technologies (PAT).

Crystalline comes with up to 8 particle detection cameras, allowing for in-situ microscopy that shows the particle size, particle shape, crystal morphology and polymorphic form. This helps accelerate the drug formulation development all while using just 2.5 to 5 mL of material in each of the 8 reactors.
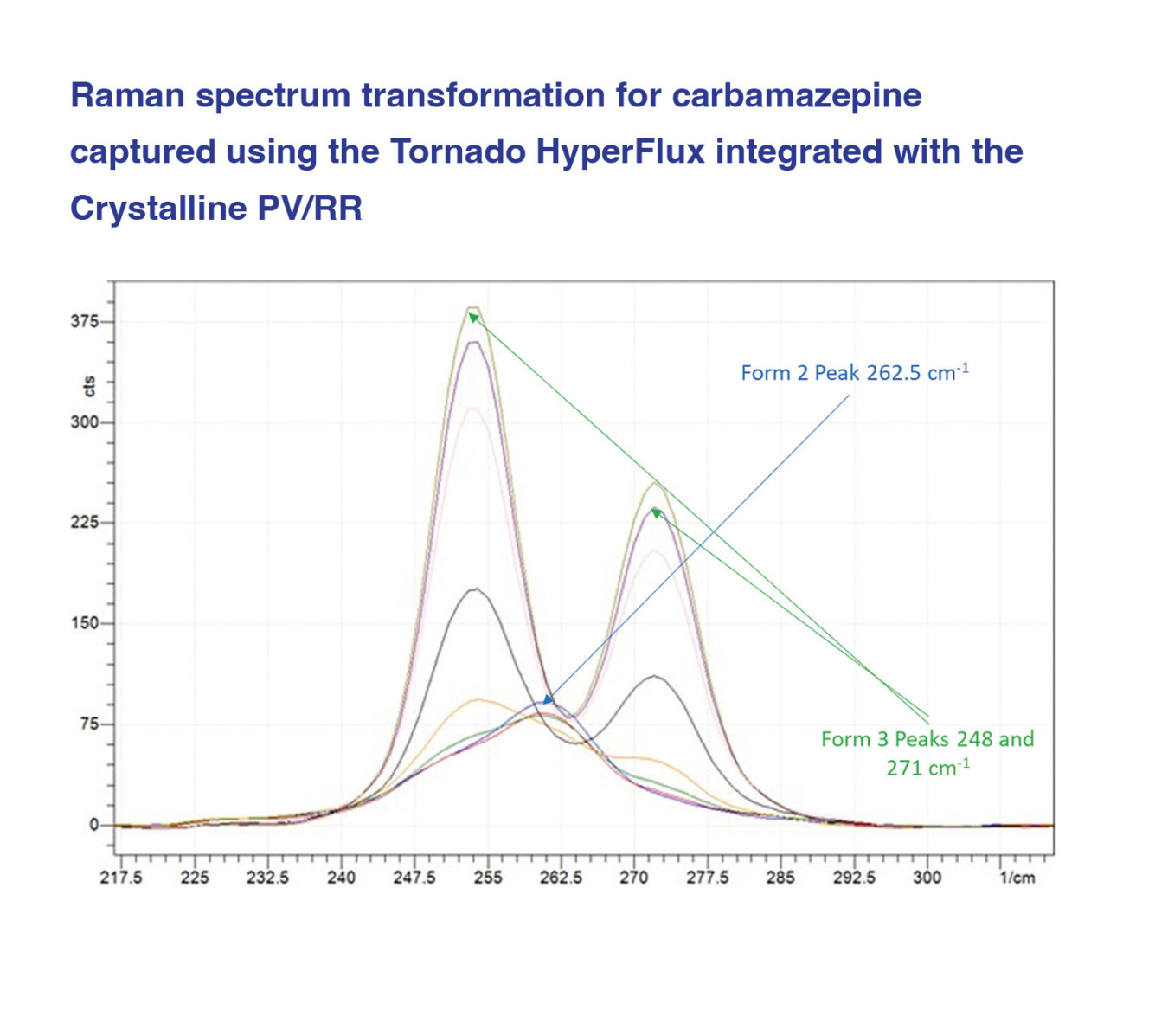
The Crystalline can be customized to allow for Raman coupling. By utilizing the Crystalline device coupled with a Raman spectrometer, it is possible to monitor and track polymorphism and morphology at low working volumes. Typically, the smallest commercial vessel where Raman spectroscopy and in-solution microscopy can be used as PAT tools is 100 mL, but by using the experimental setup with Crystalline, it is possible to obtain the same data while working at scales as low as 2.5 ml. By working at these reduced volumes, the required material required for crystallization process development can be significantly reduced. Solid form selection and research can be undertaken earlier in API development, increasing the speed they can reach manufacturing and become available to the patient.

Crystallization can be separated into two processes: nucleation and crystal growth. Nucleation is important to control, predict and understand as it plays a significant role in the polymorphic form, particle size distribution and downstream particle properties. The applicatio note discussed how to measure secondary nucleation through a single crystal seeding approach.