
Crystal16
The new industry standard
Using the Crystal16, you can acquire solubility data and meta stable zone width (MSZW) data faster and easier than with traditional methods. Upgrade your solid-state screening and solid form processes, today.
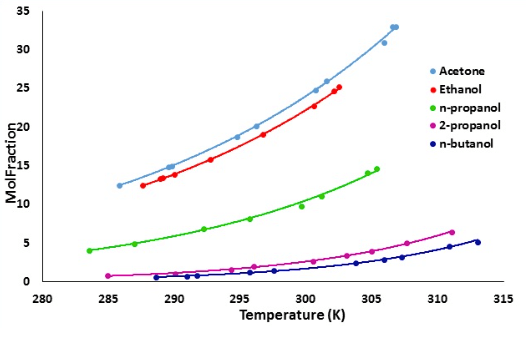
A solubility curve shows how the solubility of a substance varies with temperature. This data is used to make crucial decisions from the earliest stages of drug discovery and throughout the entire development process. Substances are typically free bases or salts, and data usually demonstrates their ability to dissolve in water and other industry accepted solvents
Experimental determination of solubility curves traditionally relies on labour-intensive, time-consuming techniques such as HPLC, which often limits the complexity of available solubility data. However, thanks to increased automation, gathering solubility data is becoming easier. The latest crystallization instruments allow users to set up their desired experiment, then leave the instrument to run the experiment independently knowing the data will be there, ready to collect and analyse, when they return.

The Crystal16™ from Technobis Crystallization Systems combines automation with integrated turbidity measurement to determine cloud and clear points, resulting in solubility data at an early stage in discovery. This instrument uses minimal sample volumes and is well suited to acquiring solubility at the early stages of drug discovery processes. By using a Crystal16™ with 16 vials holding four different concentrations of a drug substance in four different solvents and applying two temperature cycles with a heating rate of 0.5°C/min and a cooling rate of 1°C/min, four solubility curves can be simultaneously measured in duplicate in half a day (4 hours). This is 20 times faster than determining solubility data manually using HPLC. The device can thus quickly generate solvent/antisolvent libraries and give you the solubility and meta stable zone width (MSZW) curves required for your research project.
The Crystal16 is the ultimate tool for solid-state research and process development. It has been designed by scientists at Technobis Crystallization Systems for scientists, meaning that it is a user-friendly multi-reactor benchtop system with intuitive software that performs medium-throughput crystallization studies at a 1-ml scale.

Following the issuing of PAT - A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, issued by the Food & Drug Administration in 2004, there has been an increasing interest in the application of process analytical technology (PAT) in crystallization process design. The use of automated methods to determine solubility curves significantly reduces operator workload compared to traditional methods, which should encourage the use of solubility data in the early stages of crystallization process development.
Discover why many pharmaceutical companies have already chosen the Crystal16 as their standard tool to determine the solubility of their drug compounds.

Solubility data are used to make crucial decisions from the earliest stages of drug discovery and throughout the entire development process. This white paper covers two dynamic methods for effective and reproducible solubility data generation: the temperature variation and solvent addition methods. These methods can be easily applied by making use of the turbidity probes integrated in the Crystal16 and particle viewer cameras of the Crystalline instruments.