The importance of solubility in designing and optimizing lithium ion batteries for the electric vehicle market
The electric vehicle (EV) market is experiencing rapid growth in recent years, with global sales reaching 450,000 and expected to increase to 8.5 million by 2025[i] despite the overall drop in car sales. Adoption of EV’s is now seen as a key factor in reducing greenhouse gas emissions from the transport sector.
A key component in EV’s are lithium ion batteries (LIB). However, they can account for up to a third of an EV’s greenhouse gas emissions[ii]. Therefore, significant research is being done to improve the specific energy of LIB as well as metal recovery from spent LIB to improve the overall cost and environmental impact of EV’s. As part of these studies, solubility is an important property used in the designing and optimizing of a recovery process.

Understanding and measuring solubility
Solubility is defined as the amount of crystalline material that can be dissolved into a specific solvent system at a given temperature and pressure. The solubility of a material is typically dependent on the temperature. Collecting solubility at several different temperatures and concentrations allows you to plot a solubility curve. The solubility curve is a crucial piece of information in process and crystallization design. With an accurate solubility curve, it is possible to design a high yield crystallization process with good impurity rejection and tight control of particle size distribution.
The temperature variation method works by creating a suspension of known concentration then heating the sample and measuring when the samples dissolves. This method also allows to measure the temperature of crystallization giving you the metastable zone. Combining several points allows solubility and metastable curves to be plotted.
Clear points were determined using combination of the Crystalline V2 camera and particle detection. Initial clear points were positioned by the software when < 10 particles/μl were detected, and cloud points were initially positioned when > 10 particles/μl. These were then fine-tuned using the images collected with the Crystallines high resolution cameras to find the image where the last and first crystals were seen for clear, and cloud point respectively.
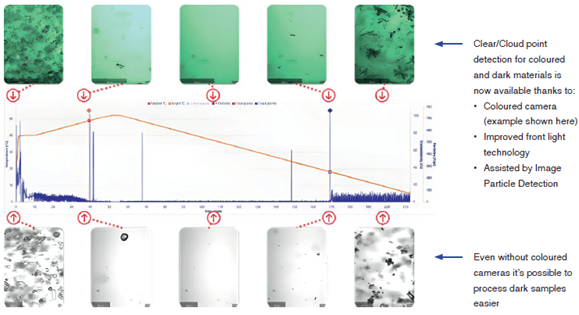
Conclusions
In conclusion, using a combination of the Crystalline’s high resolution cameras and particle detection we have successfully collected the solubility curves of coloured inorganic salts copper sulfate pentahydrate and nickel sulfate heptahydrate across a wide range of temperatures and concentrations in water. We were also able to collect the MSZW for copper sulfate pentahydrate by seeing when the number of particles detected increased, and crystals were seen on the cameras. We were able to capture the first moment of nucleation, secondary nucleation occurring and growth into pyramidal shaped crystals. Whilst we were able to detect nucleation of nickel sulfate crystals using this method, we observed two different crystal morphologies, needle and pyramidal blocks. These different crystal morphologies have been attributed in the past to different hydrate formation.
References
[i] Outlook, I.G.E., 2021. Accelerating ambitions despite the pandemic. International Energy Agency: Paris, France.
[ii] Olumide Winjobi, Jarod C. Kelly, Qiang Dai, Sustainable Materials and Technologies 32, (2022) e00415 doi:10.1016/j.susmat.2022.e00415
Curious to learn more?
Read the full application note "Solubility and crystallization of coloured compounds captured with high resolution camera".