Solubility whitepaper
The solubility of a compound in organic solvents or water is equally important for screen design and later for process development. For designing a screen, for instance around cooling crystallization, you need to select solvents that have sufficient solubility and that have a high dependency of solubility on temperature. This post covers two dynamic methods for effective and reproducible solubility data generation: the temperature variation and solvent addition methods. These methods can be easily applied by making use of the turbidity probes integrated in the Crystal16 and particle viewer cameras of the Crystalline instruments.
Solubility
Solubility is defined as the equilibrium amount of a crystalline compound that can be dissolved in a specific solvent system at the given process conditions, of which the temperature is often the most influential parameter. For many compounds, the solubility increases with temperature. Additionally, a compound may have considerably different solubility depending on the investigated solvent/solvent system.
Solubility data are used to take critical decisions from the earliest stages of drug discovery, throughout the entire process development and up to formulation. For many products, crystallization is used for purification as well as particle formation. In crystallization of active ingredients (AI), the solubility curve helps to choose a suitable crystallization process (e.g. cooling or evaporative crystallization) and determines the yield. Therefore, knowledge of the solubility is essential for the design of the crystallization process. Measuring the solubility requires accurate control of temperature and sharp observation of the phase transition, i.e. full dissolution of the solid phase, combined with information regarding the composition of the system. Since generally a reproducible solubility dataset over a temperature or compositional range is required, many data points need to be determined separately. This can be labor intensive and time consuming. Crystal16 and Crystalline instruments offer invaluable tools to automate the execution of solubility measurements in quick, controllable and reproducible manner.
Equilibrium Concentration vs Dynamic Methods
A widely accepted and accurate method for measuring the solubility is through equilibration of a suspension, followed by an assessment of the solution composition, from which the solution concentration can be determined. The method requires sampling followed by filtration to remove the solids and measurement of the concentration using for example, a gravimetric, spectroscopy or a chromatographic method like HPLC (Figure 1a). However, this Equilibrium Concentration (EqC) method is laborious and time consuming.
Two other methods are available and easily accessible: the Temperature Variation (TV) method and the Solvent Addition (SA) method, in which respectively the temperature of the suspension and the composition of the suspension are gradually changed until all crystals are dissolved. The point in the concentration-temperature diagram at which the suspension turns into a clear solution is called the clear point. A clear point temperature can be determined using the TV method. Figure 1b shows the principle of a Temperature Variation (TV) measurement, in which crystals in a suspension dissolve upon heating. At a specific temperature, the clear point temperature, crystals are not detected anymore. The SA method can be used to determine a clear point composition at a constant temperature. Figure 1c shows the principle of a Solvent Addition (SA) measurement,[i] in which the crystals in a suspension are dissolved upon dilution.
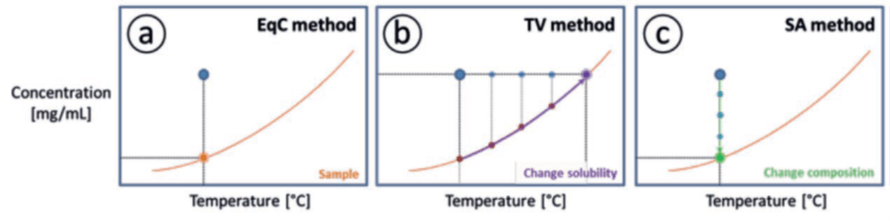
Figure 1 Three different methods of determining the solubility of a compound from a suspension with composition
The clear point can be assumed to be equal to the solubility if the heating or addition rate is chosen sufficiently small.[ii] Compared to the equilibrium concentration method, these dynamic methods are beneficial since they are much less labour intensive, much faster and have less risk of human error due to fewer required operations (no sampling or filtration). Apart from the heating or addition rate and the accuracy of the clear point determination, the error in the solubility measurements depends on the chemical system. For example, if crowning occurs, i.e. crystallization on the wall of the measurement vessel above the liquid level, the measured solubility deviates from the actual value, since part of the solid phase is not dissolved.
Moreover, the Temperature Variation (TV) and the Solvent Addition (SA) methods showed to give reliable and reproducible data in short time for a considerable amount of systems and reflected in the high number of scientific articles published per year.
The TV method is the most suitable method for determining the temperature dependent solubility line of a compound in a solvent. Upon heating a suspension of known composition, the temperature at which all crystals are dissolved marks a point on the solubility line. After a recrystallization step through cooling, the measurement can be repeated. An additional benefit of these cyclic measurements is that during the cooling stage the temperature at which the first crystals reappear can be recorded as the cloud point. The collection of cloud points gives the metastable zone width (MSZW), which is used to determine the operation range of the process and indicates the tendency towards primary nucleation. With the Crystal16, one can perform 16 solubility measurements at 1 mL scale. Measuring multiple samples simultaneously gives a dataset of saturation temperatures at different concentrations that represent the solubility line.
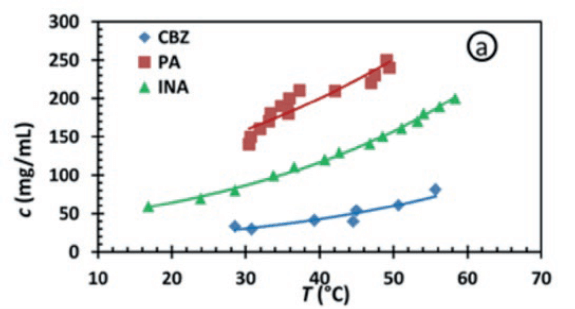
Figure 2 Solubility curves of CBZ, PA and INA in ethanol
When solubility data is required at constant temperature, which is often the case in multicomponent mixtures, SA is the method of choice. Additionally, the method is very useful for systems in which the solubility is not strongly dependent on temperature or where the MSZ is wide. However, the MSZW is not measured in this method. In the solvent addition method, the temperature is kept constant. Upon dilution of a suspension of known composition by the addition of solvent, a clear point is detected when the equilibrium concentration is reached. The clear point can be detected by a decrease in solution concentration or by the disappearing of crystals. The latter is shown in Figure 4, where a Crystalline instrument is used to monitor the suspension by using the particle viewer cameras. The cameras take pictures at regular intervals and the clear point is determined as the first picture without crystals. Therefore, a shorter time between pictures increases the accuracy of the clear point. The most important parameter in the SA method is the addition rate. It must be chosen low enough for the dissolution to occur in time.
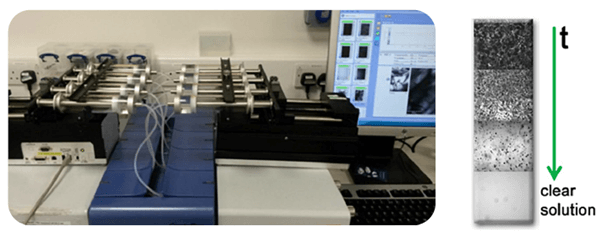
Figure 3 (a) Solvent addition using the Crystalline with 8 syringes attached. Each syringe can contain a different solvent composition. (b) The cameras of the Crystalline register the disappearance of the crystals as solvent is added over time.
Conclusion
The use of automated clear cloud point techniques, either through turbidity or camera analysis, has greatly intensified the acquisition of solubility data. The clear cloud point method for solubility determination with the Crystal16 and its integrated turbidity sensors enables scientist to easily obtain and reproduce their data, without too much effort when compared to the EqC method. The solvent addition method applied with the Crystalline and its particle viewer cameras is a popular technique for solubility determination at constant temperature and fast generation of isothermal phase diagrams. As a result, these methods are widely applied in many laboratories around the world both industry and academia. About 25 scientific articles are written each year where the use of Crystal16 and Crystalline in such measurements is emphasized.
[i] Reus, M.A.; A.E.D.M. van der Heijden; J.H. ter Horst; Org. Process. Res. Dev. 2015, 19 (8), 1004-1011.
[ii] Vellema, J.; Hunfeld, N.G.M.; Van den Akker, H.E.A.; ter Horst, J.H.; Eur. J. Pharm. Sci. 2011, 44, 621-626